TECFIDERA effectiveness
Understanding the potential benefits of Tecfidera® (dimethyl fumarate)
In separate 2-year clinical studies of patients with relapsing-remitting MS, TECFIDERA was tested against a placebo, or "fake" pill—a standard way to measure if a drug works as expected. In the first trial, 410 people took TECFIDERA and 408 took placebo (“a fake pill”). In the second trial, 359 people took TECFIDERA and 363 took placebo.
Relapses, also called flare-ups or exacerbations, can be disruptive. Reducing the risk of relapses should be one of the goals of treatment.
Although no relapsing MS medication completely gets rid of relapses, TECFIDERA cut relapses when compared with placebo.
TECFIDERA cut the risk of relapses

Study 1
27% of people taking TECFIDERA had a relapse, compared with 46% taking placebo

Study 2
29% of people taking TECFIDERA had a relapse, compared with 41% taking placebo
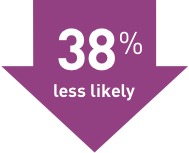
TECFIDERA cut the number of relapses by nearly half

Study 1
TECFIDERA cut the number of relapses by 53% compared with placebo
(TECFIDERA 0.172, Placebo 0.364)

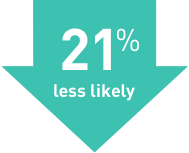
Study 2
TECFIDERA cut the number of relapses by 44% compared with placebo
(TECFIDERA 0.224, Placebo 0.401)
The link between brain lesions and the progression of MS has not been confirmed. However, brain lesions can happen without you feeling them, and may be a sign that the disease is active. Lesions revealed on an MRI scan may help your healthcare provider determine how well your treatment is working. Talking to your healthcare provider about the results of your MRI could help with the management of your relapsing MS.
TECFIDERA slowed the development of brain lesions
To understand the impact of TECFIDERA on brain lesions, researchers looked at lesions using 3 different MRI techniques to determine the age and stage of the lesions. Based on all 3 measures, people taking TECFIDERA had fewer lesions compared with those
taking placebo.
Study 1
ACTIVE INFLAMMATION
(Average number of Gd+
lesions at 2 years)
1.8
Placebo
0.1
Tecfidera
Gd+ lesions:
Inflamed brain tissue that is attacked and considered “active.” These lesions disappear when inflammation decreases.
90%
fewer Gd+ lesions for TECFIDERA
LONG-TERM IMPACT OF INFLAMMATION
(Average number of new or newly enlarging T2 lesions over 2 years)
17.0
Placebo
2.6
Tecfidera
T2 lesions:
Scars that indicate the long-term impact of MS on the brain. They can either be new lesions or old lesions that develop again.

85%
fewer T2 lesions for TECFIDERA
POSSIBLE
PERMANENT DAMAGE
(Average number of new
T1 lesions over 2 years)
5.6
Placebo
1.5
Tecfidera
T1 lesions:
Nerve cells in the brain that can’t be repaired, which can mean a loss of function.

72%
fewer T1 lesions for TECFIDERA
Study 2
ACTIVE INFLAMMATION
(Average number of Gd+
lesions at 2 years)
2.0
Placebo
0.5
Tecfidera
Gd+ lesions:
Inflamed brain tissue that is attacked and considered “active.” These lesions disappear when inflammation decreases.

74%
fewer Gd+ lesions for TECFIDERA
LONG-TERM IMPACT OF INFLAMMATION
(Average number of new or newly enlarging T2 lesions over 2 years)
17.4
Placebo
5.1
Tecfidera
T2 lesions:
Scars that indicate the long-term impact of MS on the brain. They can either be new lesions or old lesions that develop again.

71%
fewer T2 lesions for TECFIDERA
POSSIBLE
PERMANENT DAMAGE
(Average number of new
T1 lesions over 2 years)
7.0
Placebo
3.0
Tecfidera
T1 lesions:
Nerve cells in the brain that can’t be repaired, which can mean a loss of function.

57%
fewer T1 lesions for TECFIDERA